Our news
TRANSFORMATION OF SMES THROUGH INNOVATION
🏆We are pleased to announce that our company has been selected as the winner of the France 2030 regionalized call for projects in Auvergne-Rhône-Alpes region, focusing on "Transformation of SMEs through Innovation". It relates to our project on the Performance and Durability of Antimicrobial Products.
🤝We would like to thank the Région Auvergne-Rhône-Alpes and the competitiveness clusters Lyonbiopôle Auvergne-Rhône-Alpes, POLYMERIS, and TECHTERA for their support.
🔬Our company has been providing a trial service focused on assessing antiviral and antibacterial performance of antimicrobial coatings and materials that can be found today in a wide range of products (floor coverings, paint, screen protection film, switches, handrails, bank notes, etc.).
🔬To meet the needs of manufacturers and upcoming regulatory requirements, this project aims to complement our trial service with a technical offering to assess the durability of antimicrobial products throughout their life cycles. It will involve conducting laboratory-based scenarios simulating mechanical and chemical deterioration, representative of actual product usage conditions. This unique technological offering integrates both performance and durability tests within a single laboratory, will enable us to provide comprehensive and specific technical support for each type of product and technology, flexibility in project management and the possibility of developing and carrying out wear scenarios in the presence of microorganisms.

Evaluating the efficacy of surface disinfectants
VirHealth is pleased to announce the implementation of new trial procedures aimed at evaluating the efficacy of surface disinfectants, whether used with or without mechanical action (wipes),
as well as surface disinfection systems through airborne methods, against Adeno-Associated-Virus (AAV) vectors used in gene therapy.
By integrating molecular biology tools into the standardized procedures EN14476, EN16777, EN17111, EN16615, and EN17272, it is now possible to validate products, equipment, and cleaning/disinfection procedures used in production and R&D sites reproducible in laboratory settings.

SURFACE DISINFECTION NEW TEST BENCH
VirHealth has developed a new test bench to meet the requirements of EN17272 (2020, airborne disinfection system) for small-enclosure efficacy tests of 2 m3.
Also, this tool allows us to carry assays to evaluate virucidal and microbicidal activities on surfaces of passively diffused products (alcohol, essential oils, etc.).

SURFACES AND MATERIALS ANTIVIRAL ACTIVITY
We are pleased to announce the publication of the study "Antiviral Activity of Active Materials: Standard and Finger-Pad-Based Innovative Experimental Approaches" in the international review Materials.
This study was carried out in partnership with the International Centre for Infectiology Research (CIRI Centre International de Recherche en Infectiologie, Virpath team). It presents comparative data from different experimental procedures and allows defining antiviral activities of several active materials and coatings against pathogenic enveloped and non-enveloped viruses. This publication also presents an innovative experimental approach to evaluate the antiviral activities of active materials against finger-pad viral contamination. This approach is part of VirHealth's overall strategy to develop and deliver innovative laboratory trial solutions that simulate the contamination of materials and coatings in real-life conditions.
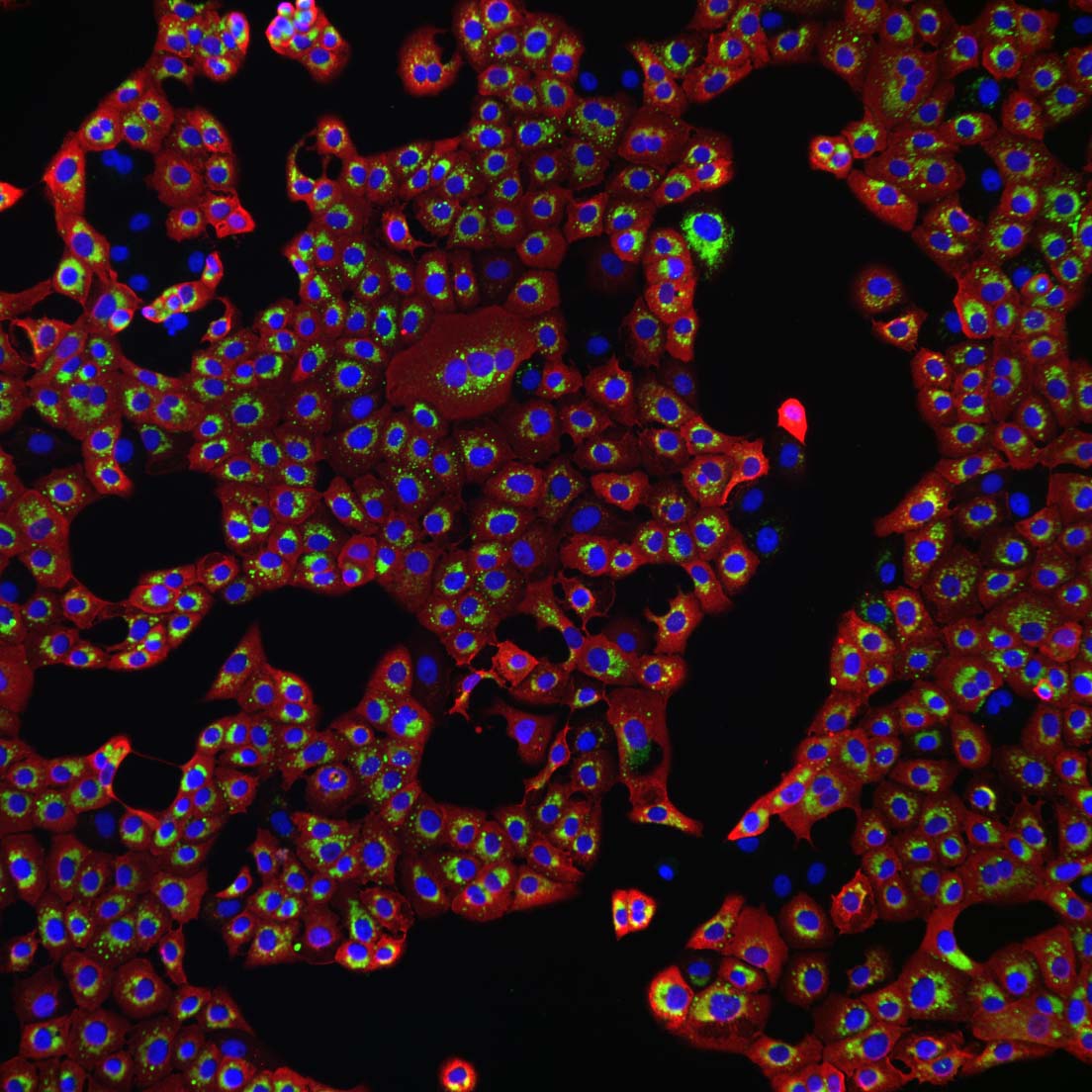
Partnership with NeoVirTech!
We are pleased to announce our partnership with NeoVirTech!
NeoVirTech and VirHealth join their virology expertise to provide customers one of the most extended virus disinfection measurement and R&D platform in France and in Europe. This alliance will allow the partners to combine their
respective expertise in an integrated offer dedicated to increase knowledge in virus persistence in the environment, virus disinfection procedure and to provide customer state of the art models for the measurement of disinfection
procedures using innovative and standardized approaches.
SURFACES AND MATERIALS ANTIVIRAL ACTIVITY
Surfaces with antiviral activities participate in the management of viral contamination by reducing
the infectious virus load over time. We offer standardized or adapted protocols to evaluate the
antiviral efficacy of porous and non-porous surfaces on a large panel of viruses (enveloped and non-
enveloped) including alpha/beta coronaviruses.

SARS-COV-2 STABILITY ON SURFACE AND DISINFECTANT
Our last preprint on the role of interfering substances on SARS-CoV-2 surface stability and virucidal efficiency of hand sanitizers and disinfectants is now available in MedRxiv.
https://www.medrxiv.org/
Contaminated environmental surfaces are considered to represent a significant vector for hospital-acquired viral infections and indirect contact transmission involving these surfaces could be the predominant transmission route for respiratory viruses. In collaboration with #VirPath, #VirHealth has used both EN14476 and EN16777 standardized protocols for evaluation of virucidal activity using tailor-made interfering substances corresponding to artificial mixture containing nasal mucus and saliva.
They reflect current “real life” conditions of use by considering the impact of omnipresent secretions from coughing or sneezing. In this study, we have measured surface stability of SARS-CoV-2 and other coronavirus in different experimental conditions and demonstrated that interfering substances (nasal mucus, saliva) play an important role in the virucidal efficiency of disinfectants.
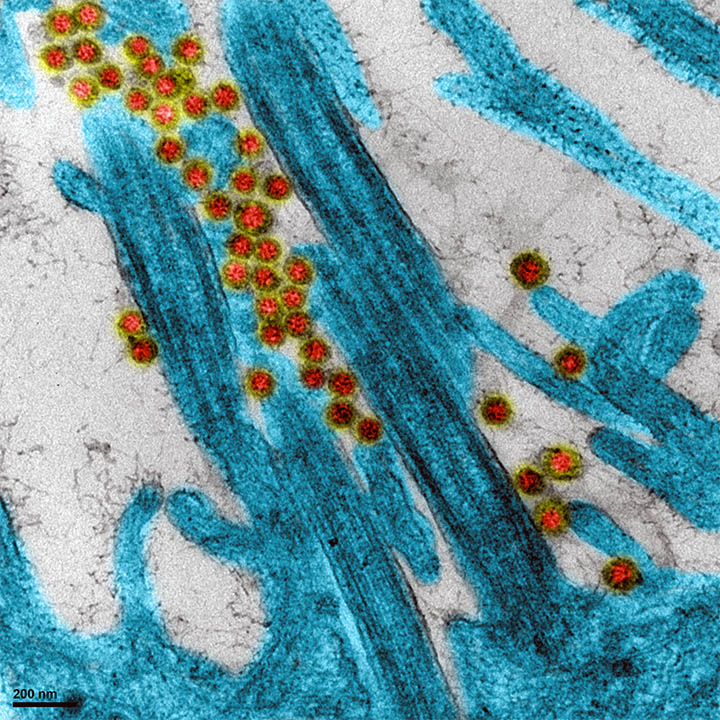
SARS-COV-2 (COVID-19)
Since 2015, VirHealth has been working in partnership with Virpath laboratory (Dr Rosa-Calatrava,
CIRI, Lyon) as part of a research and innovation program in order to answer the institutions and
manufacturers needs during health crises and viral epidemics/pandemics (Avian Influenza, MERS- CoV coronavirus,…).
Since March 2020, we have been jointly carrying out virucidal activity tests of disinfection products and equipment on Sars-Cov-2 (COVID-19). We also lead applied research programs to better understand
the virus biology and offer technical approaches for setting up appropiate testing strategies.
Photo credit : Virpath (CIRI), Dr Rosa-Calatrava.